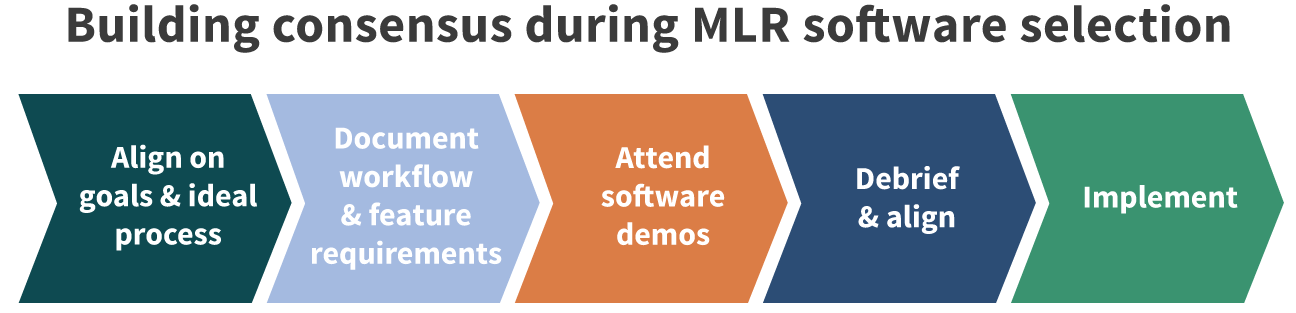
Considering a new medical, legal, regulatory review software solution at your pharma or device company is a big undertaking. Involving key stakeholders in this process is essential for success and a smooth transition to the new system.
While marketing and regulatory teams often lead the promotional review process, it’s critical to include your medical affairs team in the system selection and MLR process design. Medical affairs teams need to have a system and process that enables them to ensure medical and scientific accuracy of materials, as well as safety and efficacy data. Therefore, their insights into what’s specifically needed from an MLR solution will be invaluable to selecting the system that is right for your company and how that process is carried out at your life science organization.
Let’s take a closer look at the three key reasons to include medical affairs in your MLR system selection and process design.
Create buy-in for a new system up-front
Medical affairs teams are often considered the “power users” of an MLR system because the medical literature and claims review process is often very labor intensive for the medical team. When in the early stages of selecting a new MLR tool, different users have different perspectives on the need and what is most important from the new solution. Engaging and securing early buy-in from your medical affairs team will lead to better system adoption, smoother day-to-day use, and greater awareness of system functionality.
To further create buy-in for an MLR tool, be sure to include medical affairs as part of the demo process. It will allow them to ask specific questions for their role, see how the system works, and will ensure that their needs are going to be met by the MLR systems being considered. In addition, consult medical affairs when developing timelines for implementing a new software solution to ensure it doesn’t overlap or interfere with any planned initiatives.