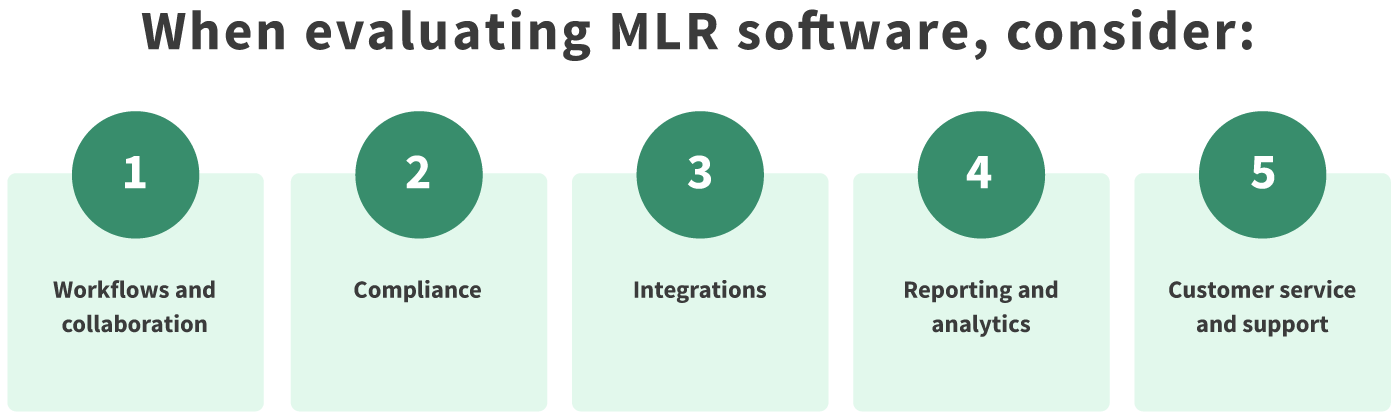
Workflows & Collaboration
Workflow optimization is one of the most fundamental parts of a contemporary MLR solution. It distinguishes outdated solutions from today’s smart software which actively removes hurdles and speeds up your MLR approval process. When looking at a potential MLR solution, ask yourself about the following workflow and collaboration-related features:
- Are you able to configure multiple workflows with varying roles, responsibilities, and permissions?
- Are you able to reconfigure workflows as requirements evolve or your business changes?
- Does the solution support multiple review types, such as promotional, medical, reference, etc.?
- Does the solution have the ability to set due dates for workflows based on project properties and requirements?
- Does the solution have the ability to annotate on a document and link to supporting materials?
- Does the solution enable side by side version comparison with previous versions of a document?
- Does the solution allow you to review and annotate directly on videos and websites in their native formats?
Compliance
For life sciences, compliance features are a necessity. Without specific compliance features, you’re not only putting your organization at risk but you’re also taking on the giant burden to fully validate generic tools for adherence to regulatory requirements and intended use. To evaluate the strength of a solution’s compliance measures, ask the following questions:
- Does the solution allow the capture of electronic signatures for approvals in compliance with FDA 21 CFR Part 11?
- Are all system and user actions captured in an audit trail that records information such as what information was changed and who changed the information?
- Does the system suggest references for you based on previous reference usage?
- Is the solution validated and come with a prepared validation package from the solution provider?
Integrations
Software packages change, tech stacks evolve, and being able to make multiple solutions work together to meet your needs will always be a priority. Remember to ask:
- Does the solution come with an API that connects to existing downstream systems in use?
Reporting & Analytics
Metrics are the foundation of process improvement. An MLR solution that lets you understand process metrics like review times and agency performance gives you meaningful insights into where you need to focus improvement efforts. When evaluating reporting functionality, ask:
- Does the solution provide out of the box metrics and reports?
- Are the reports downloadable/exportable?
Customer Service & Support
MLR solutions are not “set and forget” software packages. Support at every step of the implementation and throughout the ongoing business relationship are critical to getting value out of your tool. As your business changes, so should your MLR approval solution to accommodate. When looking at support offerings, ask:
- Are all implementations and configurations handled by the solution provider?
- Do customers get their own dedicated Customer Success Manager?
- Is 24/7 end user support available?
Get even more expert guidance on selecting an MLR tool
The right MLR solution is the key to turning what can be a cumbersome and confusing process into a fine-tuned, focused fixture of your operations. In order to help you gather all the information you need while considering this investment, download our robust promotional review system evaluation template. The template includes a robust list of criteria to evaluate your MLR solution options. Download the template and start evaluating today.