- Platform
- Platform Overview
- Life science compliance
- Enterprise-grade security
- Integrations
- Partner Ecosystem
- Workflow management
- Claims & evidence management
- Digital asset management
- Web annotation
- Health authority submission support
- Portals
- Cross channel publishing
- Global commercialization
- Vodori 360
- Reporting & analytics
- Solutions
- Resources
- Company
- Trust
Establish clear, compliant content management practices with Vodori
With Vodori, emerging life science companies establish clear and compliant content management practices–increasing speed-to-market of critical communications before, during, and after their first product launch.
Designed to propel your company towards growth
.png?width=512&height=345&name=Tab%203%20(2).png)
Easy to learn and use
Vodori's user-friendly interface enables your team to collaborate on corporate, medical, and commercial content reviews quickly–putting more time back in their day to focus on other projects. For a growing company who needs to quickly onboard new employees, it’s important to choose an MLR solution that will enable easy adoption of the technology as review accountability shifts and changes.
-
New users get started with just one 60 minute training
-
Designed with UX and UI best practices
-
Modern MLR solution with features purpose-built for life sciences
.jpg?width=512&height=345&name=Image%20%23V1%20Hero%20(1).jpg)
Expert attention from customer success
Our team is known for being responsive and a true partner for all customers, regardless of size. As you experience growth and change, the Vodori team will assist by advising on best practices, answering questions, and making any configuration changes to our software to align with your new business needs at no additional charge. We’re here to anticipate your needs and ensure you’re successful with our products.
-
Ongoing access to our Customer Success Team
-
Advice on SOPs and industry best practices
-
24/7 global support
.png?width=512&height=345&name=Tab%204%20(1).png)
Compliance for today… and tomorrow
Vodori automates compliance through configurable workflows and serves as one source of truth for your commercial content. Vodori is also a validated system with SOC 2 Type II attestation. Our rigorous compliance program covers data security, SDLC and infrastructure, risk management, and more, so as your company matures, we have you covered.
-
e-Signatures in compliance with 21 CFR Pt 11 & EU Annex 11
-
Downloadable audit trail
-
FDA submission package
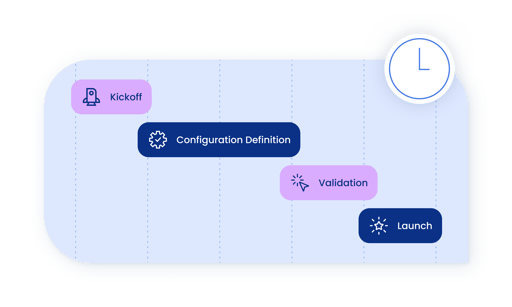
Fast, simplified implementations
At your size, you don’t have time for a drawn out implementation project. Emerging life science companies can get up and running with Vodori in as little as one week. Every part of the implementation process–from kickoff to training and launch–is handled by the Vodori team.
-
Get up and running in as little as one week
-
Minimal time investment from your team
-
Industry standard workflows
.png?width=512&height=345&name=Tab%203%20(2).png)
Easy to learn and use
Vodori's user-friendly interface enables your team to collaborate on corporate, medical, and commercial content reviews quickly–putting more time back in their day to focus on other projects. For a growing company who needs to quickly onboard new employees, it’s important to choose an MLR solution that will enable easy adoption of the technology as review accountability shifts and changes.
-
New users get started with just one 60 minute training
-
Designed with UX and UI best practices
-
Modern MLR solution with features purpose-built for life sciences
.jpg?width=512&height=345&name=Image%20%23V1%20Hero%20(1).jpg)
Expert attention from customer success
Our team is known for being responsive and a true partner for all customers, regardless of size. As you experience growth and change, the Vodori team will assist by advising on best practices, answering questions, and making any configuration changes to our software to align with your new business needs at no additional charge. We’re here to anticipate your needs and ensure you’re successful with our products.
-
Ongoing access to our Customer Success Team
-
Advice on SOPs and industry best practices
-
24/7 global support
.png?width=512&height=345&name=Tab%204%20(1).png)
Compliance for today… and tomorrow
Vodori automates compliance through configurable workflows and serves as one source of truth for your commercial content. Vodori is also a validated system with SOC 2 Type II attestation. Our rigorous compliance program covers data security, SDLC and infrastructure, risk management, and more, so as your company matures, we have you covered.
-
e-Signatures in compliance with 21 CFR Pt 11 & EU Annex 11
-
Downloadable audit trail
-
FDA submission package

Fast, simplified implementations
At your size, you don’t have time for a drawn out implementation project. Emerging life science companies can get up and running with Vodori in as little as one week. Every part of the implementation process–from kickoff to training and launch–is handled by the Vodori team.
-
Get up and running in as little as one week
-
Minimal time investment from your team
-
Industry standard workflows
“We chose Vodori at Orphazyme because the offering is relevant, their size is attractive, their price is competitive, and their responsiveness has been excellent. It was the right choice for us as an emerging ultra-rare company.”
Patrick Lennon VP, Analytics & Operations, Orphazyme























.png?width=170&height=57&name=Untitled%20design%20(8).png)





-1.png?width=170&height=57&name=sight-sciences-logo@2x%20(1)-1.png)